Quality System Certificates
Notified Body Migration of Certificates
G&H Medical Device Directive 93/42 EEC
ODP Medical Device Directive 93/42 EEC
OrthoClub Medical Device Directive 93/42 EEC
FlexMedics Medical Device Directive 93/42 EEC
Notified Body Migration of Certificates
G&H Medical Device Directive 93/42 EEC
ODP Medical Device Directive 93/42 EEC
OrthoClub Medical Device Directive 93/42 EEC
FlexMedics Medical Device Directive 93/42 EEC
 |
Catalog Number / “Reference” This symbol immediately precedes the part number of the product. |
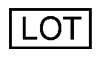 |
Batch Code / “Lot” This symbol immediately precedes the lot number of the product. G&H uses this for part traceability. |
 |
Authorized Representative in the European Community / “EU Rep” This symbol immediately precedes the name of our representative within the member states. |
 |
Do Not Reuse / “Single Use Only” Devices with this symbol are intended for single use. |
 |
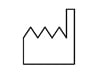
Use By / “Hourglass” This symbol identifies that the part has a "Use by" date. The part should be used prior to the date located next to this symbol. Date format YYYY-MM-DD, i.e. 2018-03-08. |
 |
Manufacturer / “Filled Factory” Symbol for Manufacturer. May also include the date of manufacture in date format YYYY-MM-DD. |
 |
Manufactured Date / “Open Factory” Date of manufacture listed under or beside this symbol in YYYY-MM-DD format. Note: Used only when the “filled factory” symbol is not present. |
 |
Consult Instructions for Use / “IFU Book” For products with additional instructions inside the package. |
 |
Consult electronic Instructions for Use For products with additional instructions electronically. |
 |
Caution/Warning Consult Instructions for use for important cautionary information such as warnings and precautions not otherwise presented on the label. |
 |
Keep away from Sunlight / “Sunlight Warning” Used on elastics. Product may demonstrate reduced performance / longevity when exposed to sunlight. |
 |
Contains latex or Presence of Natural Rubber Latex / “Latex Warning” Used on latex elastics. |
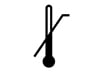 |
Temperature Upper Limit Controlled storage is required. Number indicated to the right is the max (highest) temperature at which device should be stored in, or exposed to. |
 |
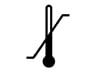
Temperature Lower Limit Controlled storage is required. Number indicated to the left is the min (lowest) temperature at which device should be stored in, or exposed to. |
 |
Temperature Limit Controlled storage is required. Number indicated to lower left is the min (lowest) temperature, and number indicated to upper right is the max (highest) temperature at which the device should be stored in, or exposed to. |
 |
Medical Device This symbol indicates the product is a medical device. |
 |
CE Mark European Conformance to Medical Device Directive 93/42/EEC or European Conformance to Medical Device Regulation 2017/745 |
2797 |
Notified Body Identification Number BSI Group The Netherlands B.V. Say Building, John M. Keynesplein 9, 1066 EP Amsterdam, Netherlands |
 |
Nickel and Chromium warning Caution: Product contains Nickel and Chromium. To alert of the potential for allergic reactions to these known allergens. |
 |
The Safe Drinking Water and Toxic Enforcement Act of 1986 / “Proposition 65” This product contains chemicals known to the State of California to cause cancer and other birth defects. |
 |
For use or distribution by orthodontic professionals only. |
 |
Data Matrix / “Barcode” Manufacturer code use to contain traceability data of the particular part. |
 |
UDI Data Matrix / “UDI Barcode” Manufacturer Unique Device Identifier “UDI” code used to contain traceability data of the particular part to meet requirement of FDA CFR 820.120 |
 |
Hook Symbol used to identify brackets that have a hook. |
 |
MR Safe Symbol used to identify product is safe to wear during MRI. |
 |
MR Conditional Symbol used to identify product is safe to wear during MRI but may affect imaging if performed above the neck. |
PRODUCT LABEL SYMBOLS & WARNINGS Doc. No. - REF.90.005.D Effective Date: 2021-07-29 |
|
Mailing Address
40 Linville Way
Franklin, IN 46131 USA
Phone
1-800-526-1026 (US & Canada)
1-317-346-6655 (International)